How Has The Model Of An Atom Changed Over Time?
How has the Model of the Atom Changed Over the Years?
The Atomic Structure
The historical development of diminutive models:
Over the concluding 100 years, scientists accept done investigations which show that atoms are fabricated up of even smaller particles.
People also ask
- How would you describe the Structure of an Atom
- What was Rutherford's Original Hypothesis
- What did Bohr Contribute to the Theory of an Atom
- What are the Characteristics of Electron, Proton and Neutron
- Explain Bohr Bury rules for Distribution of Electrons into Different Shells
- What are the Isotopes, Isobars and Isotones of an Chemical element
- What did Dalton Contribute to the Agreement of the Atom
- What is the Definition of Atom and Molecule
- What is Atomic Mass
Subatomic Particles Examples
Subatomic particles of an cantlet:
- Atoms are made upwards of three types of smaller particles, namely protons, neutrons and electrons.
- These particles are known as subatomic particles.
- The relative masses and charges of these three subatomic particles are shown in Table ii.4. The masses and charges are measured relative to a proton because their actual values are incredibly pocket-sized.
Relative masses and charges of subatomic particlesSubatomic particle Symbol Relative mass Relative electrical charge Proton P 1 + i Neutron n one 0 Electron east– i/1840 -1 - Protons and neutrons are found in the nucleus of an atom while electrons surround the nucleus.
Nucleus contains protons and neutrons
(a) As the masses of protons and neutrons are greater than electrons, most of the mass of an atom is concentrated in the nucleus.
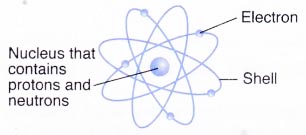
(b) The nucleus has an overall positive charge due to the positively-charged protons in information technology.
(c) An cantlet consists of an equal number of electrons and protons. Hence, an cantlet is electrically neutral.
How exercise you make up one's mind the number of nucleons?
Proton number and nucleon number:
- Scientists use the proton number and nucleon number to describe an atom.
- Proton number of an element is the number of protons in its atom.
- Since atoms are neutral, the proton number is as well the number of electrons in the atom.
- Each element has its ain proton number. For example, sodium has a proton number of 11. Hence, all atoms of sodium have eleven protons. Oxygen has a proton number of 8, and then all oxygen atoms take 8 protons.
- Nucleon number of an chemical element is the total number of protons and neutrons in its cantlet. From definition,
nucleon number = number of protons + number of neutrons
However, number of protons = proton number
Therefore,
nucleon number = proton number + number of neutrons
or
number of neutrons = nucleon number – Proton number - The nucleon number is too known every bit the mass number.
- The relative mass of an atom is almost the aforementioned as its nucleon number. The nucleon number is sometimes used as the approximate relative mass in calculations.
- Protons and neutrons are collectively called nucleons because protons and neutrons occupy the nucleus.
- In a neutral atom, number of electrons = number of protons.
Proton number and Nucleon number Example Issues with Solutions
1. A chlorine atom has 17 protons and 18 neutrons.What are the proton number and nucleon number of the atom?
Solution:
Proton number = number of protons = 17
Nucleon number= number of protons + number of neutrons
= 17 + xviii
= 35
2. Lithium has a proton number of 3 and a nucleon number of 7. How many protons, electrons and neutrons are nowadays in an atom of lithium?
Solution:
Number of protons = proton number = 3
An atom is neutral.
Therefore,
number of electrons = number of protons = 3
Number of neutrons
= nucleon number – proton number
= 7 – 3
= 4
What element is represented past the symbol N?
Symbols of elements:
- Each element is given a proper noun and a symbol. Some examples of elements and their symbols are shown in Table.
Element Symbol Element Symbol Hydrogen H Sodium Na Helium He Magnesium Mg Lithium Li Aluminium Al Beryllium Be Silicon Si Boron B Phosphorus P Carbon C Sulphur Due south Nitrogen N Chlorine Cl Oxygen O Argon Ar Fluorine F Potassium 1000 Neon Ne Calcium Ca - Discover that:
(a) each symbol consists of ane or ii letters. For elements with 2-letter symbols, the commencement letter is e'er a capital alphabetic character while the 2d letter is always a pocket-sized letter.
(b) for most elements, the letters used in their symbols take either the offset letter of the alphabet or the kickoff and another letter of their names. Some examples include the symbols of hydrogen, H; neon, Ne and magnesium, Mg.
(c) for some elements, the symbols come from Latin names such equally natrium (Na) for sodium and kalium (M) for potassium. - The standard representation for an cantlet of any element shows the proton number and the nucleon number of the element. It can be written as follows:
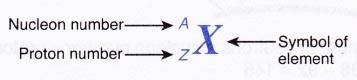
For case, a sodium atom is represented as \(_{ 11 }^{ 23 }{ Na }\). This means that sodium has a proton number of xi and a nucleon number of 23. - Sometimes an element is represented by using only the nucleon number. For example, \(_{ 11 }^{ 23 }{ Na }\) is represented by sodium-23.
Representation of Atom Instance Problems with Solutions
1. A carbon atom has half-dozen protons and vii neutrons. Correspond the cantlet in the grade of \(_{ Z }^{ A }{ X }\).
Solution:
The symbol of carbon is C.
A denotes the nucleon number.
Nucleon number = number of protons + number of neutrons
= 6 + vii = xiii
Z denotes the proton number.
Proton number = number of protons = six
Hence, the carbon cantlet is represented equally \(_{ 6 }^{ 13 }{ C }\).
2. Phosphorus-32 has 17 neutrons. What are the proton number and nucleon number of the cantlet? Stand for the atom in the form of \(_{ Z }^{ A }{ X }\).
Solution:
The symbol of phosphorus is P.
Proton number = nucleon number – number of neutrons
= 32-17 = 15
Nucleon number = 32
Hence, the phosphorus atom is represented as \(_{ 15 }^{ 32 }{ P }\).
Source: https://www.aplustopper.com/model-atom-changed-years/
Posted by: ortizfoophy.blogspot.com


0 Response to "How Has The Model Of An Atom Changed Over Time?"
Post a Comment